
by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To describe the procedure for the operation, cleaning, and maintenance of the Multimill to ensure consistent performance and compliance with cGMP requirements. 2.0 Scope This SOP applies to the Multimill installed in the production area of [Company Name],...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To describe the procedure for operation, cleaning, and maintenance of the Cad Mill to ensure consistent particle size reduction and compliance with cGMP requirements. 2.0 Scope This SOP applies to the Cad Mill installed in the production area of [Company...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To lay down the procedure for the operation, cleaning, and maintenance of the paste kettle to ensure uniform binder preparation in compliance with cGMP. 2.0 Scope This SOP applies to the paste kettle used in the production area for binder solution...
by Dr. Yashashwini Reddy | Sep 2, 2025
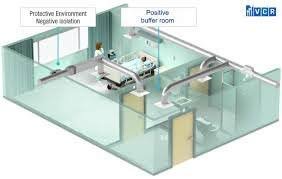
🔹 Buffer Area in Sterile Facility 1. Definition The Buffer Area (also called the cleanroom or critical area) is a Class 100 / ISO 5 / Grade A-B environment where aseptic processing, sterile filling, and direct product exposure operations are performed. It is...
by Dr. Yashashwini Reddy | Sep 2, 2025
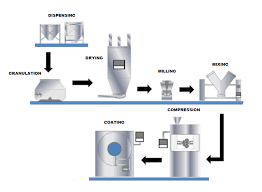
🔹 Tablet Manufacturing Process: An Overview Tablets are solid dosage forms containing one or more active pharmaceutical ingredients (APIs) with suitable excipients. The manufacturing process must ensure uniformity, stability, safety, and efficacy. 1. Pre-Formulation...







