
by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Regulatory Compliance in Pharmaceuticals: 8 Common Mistakes and How to Avoid Them Definition:Regulatory compliance in pharmaceuticals means adhering to laws, regulations, and guidelines (e.g., US FDA, EMA, WHO, MHRA, CDSCO) to ensure medicines are safe, effective,...

by Dr. Yashashwini Reddy | Aug 29, 2025
1. Purpose To define the procedure for calibration of the Slit-to-Agar Air Sampler used for environmental monitoring to ensure accurate measurement of microbial air contamination. 2. Scope This SOP applies to all STA air samplers used in microbiology or cleanroom...
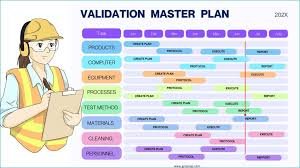
by Dr. Yashashwini Reddy | Aug 18, 2025
How to Write a Validation Master Plan (VMP) A Validation Master Plan (VMP) is a high-level document that outlines a company’s overall philosophy, intentions, and approach for establishing performance qualification, validation, and compliance of its facilities,...

by Dr. Yashashwini Reddy | Apr 30, 2025
The practice of calibrating dissolution testers with salicylic acid tablets was discontinued for several reasons: Inconsistent Results: Salicylic acid tablets showed variations in their dissolution profiles, which could result in inconsistent and unreliable...
by Dr. Yashashwini Reddy | Apr 29, 2025
Quality issues in the pharmaceutical industry can arise from a variety of sources across the entire lifecycle of drug development, manufacturing, and distribution. Below is a detailed breakdown of the root causes: 1. Manufacturing Process Failures Inadequate Process...







