by Dr. Yashashwini Reddy | Oct 9, 2025
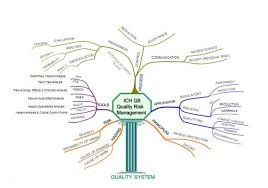
Quality Risk Management (QRM) is a systematic process for the assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. The purpose of QRM is to ensure that product quality, patient safety, and regulatory...

by Dr. Yashashwini Reddy | May 6, 2025
A Checklist to Review Tablet Process Validation is essential for ensuring that tablet manufacturing processes are consistently producing products that meet predefined specifications and regulatory requirements. Process validation in pharmaceutical manufacturing is a...
by Dr. Yashashwini Reddy | Nov 28, 2024
Why Data Integrity Matters in Pharmaceuticals: Insights from Patients and Regulators In pharmaceuticals, “data integrity” ensures data remains accurate, consistent, and reliable throughout its lifecycle. It forms the backbone of quality assurance in...
by Dr. Yashashwini Reddy | Nov 1, 2024
Disinfectant Validation in the Pharmaceutical Industry In the highly regulated world of pharmaceuticals, maintaining clean and sterile environments is essential to ensure product safety and quality. Disinfectant validation plays a critical role in this process,...
by Dr. Yashashwini Reddy | Oct 18, 2024
Importance of Recalls in the Pharmaceutical Industry: Safeguarding Public Health and Compliance In the pharmaceutical industry, ensuring the safety, efficacy, and quality of drugs is of paramount importance. Despite stringent quality control measures and regulatory...





