
by Dr. Yashashwini Reddy | Sep 13, 2025
Liquid Production Audit Checklist 1. Facility & Environment Production area designed as per GMP (segregated zones, unidirectional flow). Cleaning and sanitization records of manufacturing & filling areas available. Air Handling Units (AHUs) qualification and...
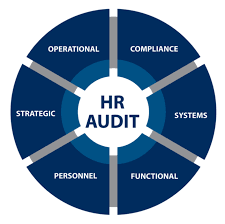
by Dr. Yashashwini Reddy | Sep 13, 2025
HR Audit Checklist 1. Recruitment & Selection Existence of recruitment policies and SOPs. Proper manpower requisition approvals before hiring. Background verification and reference checks of employees. Job descriptions and competency requirements documented....

by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Data Falsification in the Pharmaceutical Industry Definition:Data falsification is the intentional alteration, manipulation, or fabrication of data in order to misrepresent results and meet regulatory, quality, or business expectations. It is one of the most serious...

by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Contamination Issues in Pharmaceutical Production and Their Prevention Definition:Contamination in pharmaceuticals refers to the undesired introduction of chemical, microbial, or physical material into drug products during manufacturing, packaging, storage, or...

by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Computerized System Validation (CSV) in Pharmaceuticals Definition:Computerized System Validation (CSV) is a documented process of ensuring that computerized systems used in GxP (Good Practice) environments consistently function as intended and comply with...







