
by Dr. Yashashwini Reddy | Sep 15, 2025
Annual Product Quality Review (APQR/APR/PQR) in Quality Improvements 1. What is APQR/APR/PQR? Annual Product Quality Review (APQR), also known as Annual Product Review (APR) or Product Quality Review (PQR), is a regulatory requirement under ICH Q7, EU GMP Chapter 1...
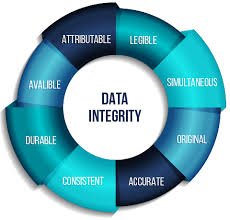
by Dr. Yashashwini Reddy | Sep 15, 2025
Audit Trail Requirements in Pharmaceuticals An audit trail is a secure, computer-generated, time-stamped record that allows for the reconstruction of events related to the creation, modification, or deletion of electronic data. In the pharmaceutical industry, audit...

by Dr. Yashashwini Reddy | Sep 15, 2025
FDA Forms Generally Used in Pharmaceutical Inspection 1. Form FDA 482 – Notice of Inspection Issued at the start of an inspection. Notifies the company that FDA investigators are officially beginning the inspection under the authority of the FD&C Act (Section...

by Dr. Yashashwini Reddy | Sep 15, 2025
Preparation for GMP Audit in Pharmaceuticals Good Manufacturing Practice (GMP) audits are crucial checkpoints that ensure pharmaceutical companies comply with regulatory standards (FDA, EMA, MHRA, WHO). Proper preparation reduces the risk of critical observations and...

by Dr. Yashashwini Reddy | Sep 15, 2025
4 Tips to Reduce 483 Observations The FDA issues Form 483 to highlight potential GMP violations observed during inspections. Minimizing these observations requires a proactive approach to compliance and quality culture. 1. Strengthen Documentation Practices Ensure...







