by Dr. Yashashwini Reddy | Sep 18, 2025
📌 User Requirement Specification (URS) in Pharmaceuticals 🔹 Definition A User Requirement Specification (URS) is a document that clearly defines what the end-user expects from a system, equipment, utility, or software before procurement, design, or implementation. It...
by Dr. Yashashwini Reddy | Sep 18, 2025
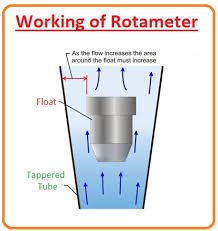
📌 Rotameter A Rotameter is a simple, reliable device used to measure the flow rate of liquids and gases in pharmaceutical, chemical, and other industries. It belongs to the variable area flowmeter family. 🔬 Principle of Rotameter Works on the variable area principle:...

by Dr. Yashashwini Reddy | Sep 16, 2025
1.0 Purpose To describe the procedure for proper cleaning, maintenance, and storage of sampling equipment used for raw materials, intermediates, and finished products to avoid contamination, cross-contamination, and ensure equipment integrity. 2.0 Scope This SOP...

by Dr. Yashashwini Reddy | Sep 16, 2025
1.0 Purpose To establish a procedure for scheduling and conducting retesting of raw materials after the initial expiry/retest period to ensure continued compliance with specifications before use in manufacturing. 2.0 Scope This SOP applies to all raw materials stored...
by Dr. Yashashwini Reddy | Sep 16, 2025
1.0 Purpose To define the procedure for the correct operation of the sampling booth to ensure clean, contamination-free, and safe conditions during the sampling of raw materials and to prevent cross-contamination. 2.0 Scope This SOP is applicable for all personnel...





