
by Dr. Yashashwini Reddy | Apr 29, 2025
The essential GMP (Good Manufacturing Practice) compliance requirements for pharmaceuticals are designed to ensure the consistent production of high-quality, safe, and effective medicinal products. These requirements are globally recognized and enforced by regulatory...

by Dr. Yashashwini Reddy | Apr 28, 2025
Resolving API Impurity Issues in Drug Development:- In pharmaceutical drug development, Active Pharmaceutical Ingredients (APIs) are the core components responsible for the therapeutic effect of a drug. However, during the manufacturing and synthesis of APIs,...

by Dr. Yashashwini Reddy | Apr 23, 2025
Standard Operating Procedure (SOP) Here is a Standard Operating Procedure (SOP) for the Operation and Calibration of a Leak Tester (typically used for checking the integrity of blister packs, strip packs, sachets, etc.). 1. Purpose To lay...

by Dr. Yashashwini Reddy | Apr 21, 2025
Standard Operating Procedure (SOP) A Standard Operating Procedure (SOP) for Reduced Testing in Quality Control typically involves guidelines for when and how to reduce the scope of testing while ensuring product quality remains intact. The goal is...
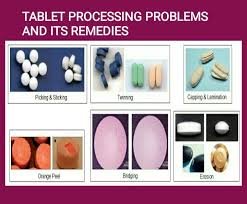
by Dr. Yashashwini Reddy | Apr 13, 2025
Tablet twinning refers to a manufacturing flaw in which two or more tablets adhere to one another, creating a “twin” or cluster during the coating phase. This issue is particularly prevalent among flat or parallel-faced tablets, primarily due to moisture...







