by Dr. Yashashwini Reddy | Aug 18, 2025
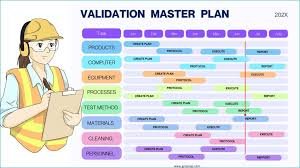
How to Write a Validation Master Plan (VMP) A Validation Master Plan (VMP) is a high-level document that outlines a company’s overall philosophy, intentions, and approach for establishing performance qualification, validation, and compliance of its facilities,...
by Dr. Yashashwini Reddy | Aug 18, 2025
Purpose of Process Validation in Pharmaceuticals Process validation is a documented evidence-based approach that ensures a manufacturing process, when operated within established parameters, can consistently produce pharmaceutical products meeting predetermined...

by Dr. Yashashwini Reddy | Aug 18, 2025
Three Consecutive Batches for Validation in Pharmaceuticals In the pharmaceutical industry, process validation is a critical requirement to demonstrate that a manufacturing process consistently produces a product meeting predetermined quality attributes. Why three...

by Dr. Yashashwini Reddy | Aug 18, 2025
Validation of Compressed Air in Pharmaceuticals Compressed air is widely used in pharmaceutical manufacturing for processes like cleaning, drying, aeration, packaging, and equipment operation. Since compressed air may directly or indirectly come in contact with...
by Dr. Yashashwini Reddy | Aug 18, 2025
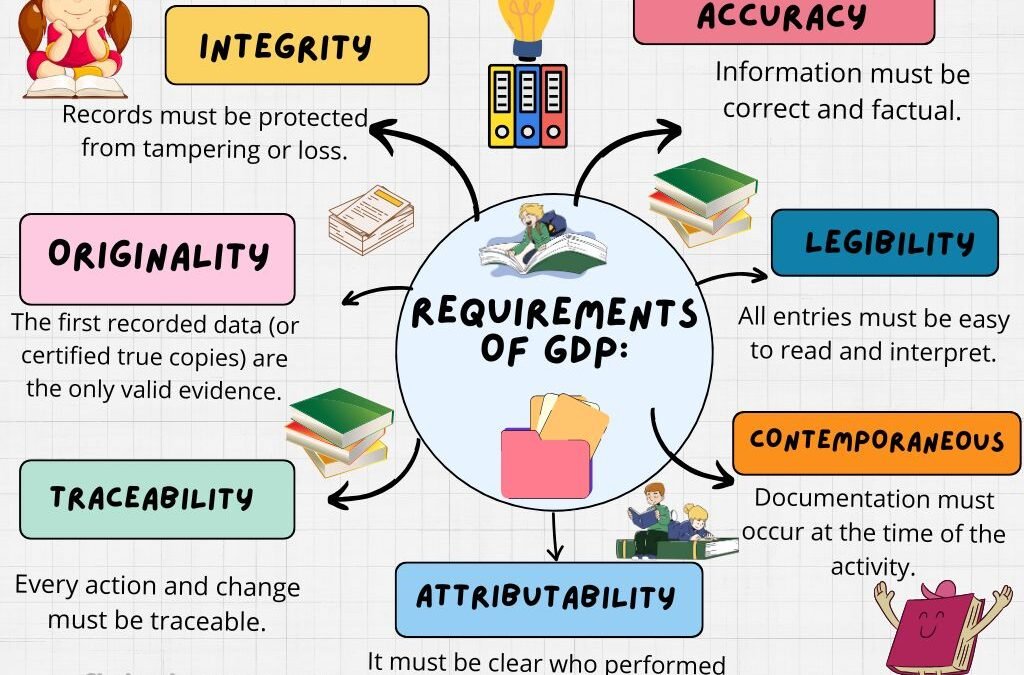
Requirements for Good Documentation Practice (GDP) Legibility All entries should be clear, readable, and permanent (no pencil or erasable ink). Accuracy Data should reflect the actual observation, measurement, or action without manipulation. Contemporaneous Recording...







