
by Dr. Yashashwini Reddy | Oct 8, 2025
🧭 Quality Guidelines – Overview Quality Guidelines are internationally harmonized standards developed mainly by the International Council for Harmonisation (ICH) and other regulatory authorities (like FDA, EMA, WHO, CDSCO).They help ensure that pharmaceutical products...
by Dr. Yashashwini Reddy | Sep 29, 2025
Advantages of Pharmaceutical Quality Audits Regulatory Compliance – Ensures adherence to GMP, FDA, EMA, WHO, and other regulatory requirements. Risk Identification – Detects potential risks, deviations, and non-compliance before they become critical issues. Continuous...
by Dr. Yashashwini Reddy | Sep 26, 2025
📌 Case Study: Sun Pharma / Caraco (US) – Recalls due to Contamination and CGMP Non-Compliance Background Caraco Pharmaceutical Laboratories, a US-based generic manufacturer and subsidiary of Sun Pharma, came under FDA scrutiny due to multiple quality issues. As one of...
by Dr. Yashashwini Reddy | Sep 24, 2025
Case Study: Ranbaxy & Glass Particles Recall (2014) Background:In 2014, Ranbaxy Laboratories recalled several batches of injectable products in the United States due to the presence of glass particles in vials. The recall was classified as a Class II recall by the...
by Dr. Yashashwini Reddy | Sep 23, 2025
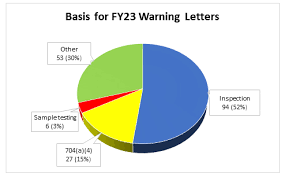
📌 Case Study: Data Integrity Failures – FDA Warning Letters 1. Background In recent years, several pharmaceutical companies (especially in India and China) have faced FDA Warning Letters and import alerts because of data integrity violations. These cases highlight the...






