by Dr. Yashashwini Reddy | Sep 2, 2025
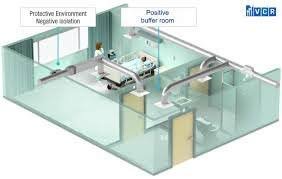
🔹 Buffer Area in Sterile Facility 1. Definition The Buffer Area (also called the cleanroom or critical area) is a Class 100 / ISO 5 / Grade A-B environment where aseptic processing, sterile filling, and direct product exposure operations are performed. It is...

by Dr. Yashashwini Reddy | Sep 2, 2025
How to calculate To calculate the capacity of tablet coating pan we need two factors which are given as below, Brimful volume of pan Tablet bulk density Bring full volume is the volume of the water which can be added in the tablet coating pan up to its brim or upper...

by Dr. Yashashwini Reddy | Sep 2, 2025
1. Influence on Stability Moisture Sensitivity: Smaller granules have a larger surface area, which can increase moisture absorption, leading to hydrolytic degradation of moisture-sensitive drugs. Oxidation: Increased surface area also accelerates oxidative...

by Dr. Yashashwini Reddy | Sep 2, 2025
Generic Drugs Manufacturing: Opportunities and Obstacles Opportunities: Cost-effectiveness: Generic drugs offer patients affordable alternatives to branded medicines, increasing accessibility and market demand. Patent Expiry of Blockbuster Drugs: As patents of many...
by Dr. Yashashwini Reddy | Sep 2, 2025
📌 Restricted Access Barrier System (RABS) in Pharmaceuticals A Restricted Access Barrier System (RABS) is an advanced containment and protection technology used in pharmaceutical sterile manufacturing to reduce contamination risks. It provides a physical and...






