
by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To lay down the procedure for the operation, cleaning, and maintenance of the paste kettle to ensure uniform binder preparation in compliance with cGMP. 2.0 Scope This SOP applies to the paste kettle used in the production area for binder solution...
by Dr. Yashashwini Reddy | Sep 3, 2025
Novel Drug Delivery Systems (NDDS) A Novel Drug Delivery System is a modern approach of formulating and delivering drugs to improve efficacy, safety, patient compliance, and targeted delivery compared to conventional dosage forms (like tablets, capsules, or...

by Dr. Yashashwini Reddy | Sep 3, 2025
Building Better Manufacturing Facilities in Pharmaceuticals Pharmaceutical manufacturing facilities must be designed to ensure product quality, patient safety, and regulatory compliance. A “better” facility goes beyond infrastructure—it integrates technology,...
by Dr. Yashashwini Reddy | Sep 3, 2025
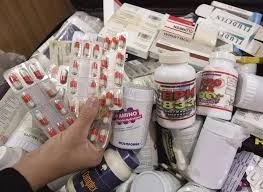
Common Causes of Low Quality in Pharmaceuticals Maintaining pharmaceutical quality is critical to ensure safety, efficacy, and regulatory compliance. Low-quality medicines can arise from multiple factors across raw materials, manufacturing, storage, and distribution....

by Dr. Yashashwini Reddy | Sep 3, 2025
Human Error: Some Fresh Approaches to Consider Human errors are often seen as a root cause in pharmaceutical and other regulated industries, but simply blaming individuals is not enough.Fresh approaches focus on: ✅ System Design – Designing error-proof processes...







