
by Dr. Yashashwini Reddy | Sep 6, 2025
Data Falsification in the Pharmaceutical Industry 1. What is Data Falsification? Data falsification in pharmaceuticals refers to the intentional manipulation, fabrication, or misrepresentation of data in order to conceal deviations, meet specifications, or mislead...
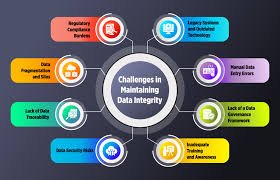
by Dr. Yashashwini Reddy | Sep 6, 2025
Importance of Data Integrity for Pharmaceutical Regulatory Agencies 1. What is Data Integrity? Data integrity means maintaining the accuracy, completeness, consistency, and reliability of data throughout its lifecycle, ensuring it is attributable, legible,...

by Dr. Yashashwini Reddy | Sep 6, 2025
The Importance of FDA Form 483s and Warning Letters in Pharmaceuticals 1. What is FDA Form 483? FDA Form 483, “Inspectional Observations,” is issued by FDA inspectors to company management at the end of an inspection. It lists potential violations of the Food, Drug,...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To lay down the procedure for operation, cleaning, and maintenance of the Cage Blender to ensure uniform mixing of powders and granules in compliance with cGMP. 2.0 Scope This SOP applies to the Cage Blender installed in the production area of [Company...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To describe the procedure for operation, cleaning, and maintenance of the Cad Mill to ensure consistent particle size reduction and compliance with cGMP requirements. 2.0 Scope This SOP applies to the Cad Mill installed in the production area of [Company...







