
by Dr. Yashashwini Reddy | Sep 8, 2025
1. Be Inspection-Ready Always Keep documentation, equipment, and facilities in a state of compliance at all times. Conduct regular self-inspections and mock audits. Ensure all records are updated, accurate, and readily retrievable. 2. Train Employees on GMP and...
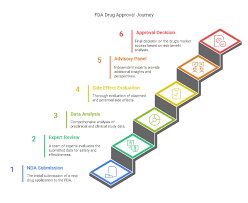
by Dr. Yashashwini Reddy | Sep 6, 2025
5 Steps of FDA Approvals The U.S. Food and Drug Administration (FDA) follows a structured process to ensure that drugs are safe, effective, and high-quality before they reach patients. 1. Preclinical Testing (Laboratory & Animal Studies) Conducted before human...

by Dr. Yashashwini Reddy | Sep 6, 2025
Requirements of FDA for Training in Pharmaceuticals Training in the pharmaceutical industry is a critical requirement under cGMP regulations (21 CFR Parts 210 & 211). The FDA expects companies to have a structured, documented training system that ensures employees...
by Dr. Yashashwini Reddy | Sep 6, 2025
Planning and Procedure Followed During Regulatory Audits Regulatory audits (by USFDA, EMA, MHRA, WHO, or local authorities) are conducted to verify compliance with cGMP and regulatory standards. Effective preparation and systematic execution are crucial for success....

by Dr. Yashashwini Reddy | Sep 6, 2025
Pharmaceutical companies operate under strict cGMP regulations enforced by agencies like FDA, EMA, MHRA, and WHO. Even well-established firms make compliance mistakes that can lead to FDA 483s, Warning Letters, recalls, or import alerts. Below are 8 of the most common...






