
by Dr. Yashashwini Reddy | Sep 9, 2025
🧾 Common FDA 483 Observations Related to Cleaning in Pharmaceuticals 1. Inadequate Cleaning Validation Cleaning validation not performed for all product-contact equipment. Worst-case product selection (hardest to clean, most toxic/potent, least soluble) not justified....
by Dr. Yashashwini Reddy | Sep 9, 2025
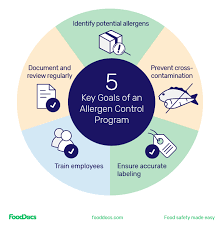
🧾 Allergen Control Plan for Pharmaceuticals 1. Risk Assessment Identify allergenic excipients (e.g., lactose, soy lecithin, egg-derived albumin, peanut oil, gluten, gelatin). Assess risk of cross-contamination during manufacturing, packaging, storage, and cleaning....
by Dr. Yashashwini Reddy | Sep 9, 2025
✅ Areas to Inspect During Self-Inspection in Pharmaceuticals Personnel & Training Verify staff qualifications, training records, and adherence to GMP practices. Premises & Facilities Check cleanliness, maintenance, pest control, HVAC systems, and environmental...

by Dr. Yashashwini Reddy | Sep 8, 2025
Recent GMP Violations at Indian Pharma Facilities 1. Granules India (Telangana) In a 2024 inspection, the FDA observed severe cross-contamination issues: residues in air ducts, microbial contamination despite HEPA filters, bird droppings and feathers in production...

by Dr. Yashashwini Reddy | Sep 8, 2025
Trends in GMP Violations in Pharmaceuticals Good Manufacturing Practice (GMP) violations remain one of the leading causes of FDA 483s, Warning Letters, and drug recalls. Recent inspection trends show recurring compliance gaps across global pharma companies: 1. Data...







