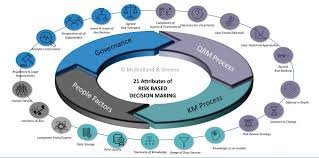
by Dr. Yashashwini Reddy | Oct 9, 2025
A Pharmaceutical Quality System (PQS) is a comprehensive framework that ensures the consistent quality, safety, and efficacy of pharmaceutical products throughout their life cycle — from development to discontinuation. It aligns with ICH Q10, which complements ICH Q8...
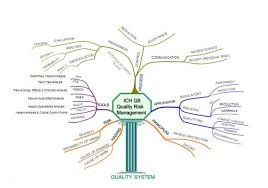
by Dr. Yashashwini Reddy | Oct 9, 2025
Quality Risk Management (QRM) is a systematic process for the assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. The purpose of QRM is to ensure that product quality, patient safety, and regulatory...

by Dr. Yashashwini Reddy | Oct 9, 2025
Good Manufacturing Practices (GMP) for APIs are essential to ensure that the Active Pharmaceutical Ingredients produced are of consistent quality and meet their intended use requirements. These guidelines are mainly described in ICH Q7 – Good Manufacturing Practice...
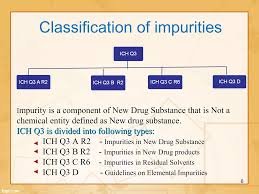
by Dr. Yashashwini Reddy | Oct 8, 2025
Elaboration:Impurities are unwanted chemicals that remain with the active pharmaceutical ingredient (API) or develop during formulation and storage. According to ICH Q3A (for new drug substances) and ICH Q3B (for new drug products), impurities must be identified,...

by Dr. Yashashwini Reddy | Oct 8, 2025
🧪 ICH Q2: Analytical Validation 🔹 Full Title: ICH Q2(R2) – Validation of Analytical Procedures 🔹 Objective: To provide guidance on the validation of analytical methods used to test drug substances and products — ensuring that the methods are accurate, reliable, and...







