by Dr. Yashashwini Reddy | Aug 9, 2025
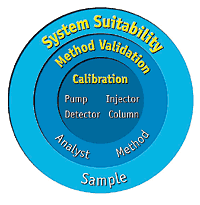
System Suitability in HPLC Analysis What is System Suitability? System Suitability Testing (SST) is a set of analytical checks performed before and during analysis to ensure that the HPLC system and method are capable of producing accurate, precise, and reproducible...

by Dr. Yashashwini Reddy | Aug 9, 2025
10 Tips for HPLC Analysis in Pharmaceuticals Choose the Right Column Select the correct stationary phase (C18, C8, phenyl, etc.) based on analyte polarity. Consider particle size, column length, and pore size for optimal separation. Prepare Mobile Phase Properly Use...
by Dr. Yashashwini Reddy | Aug 9, 2025
Difference Between C8 and C18 Columns in HPLC Parameter C8 Column C18 Column Full Name Octyl column Octadecyl column Carbon Chain Length 8 carbons 18 carbons Stationary Phase Polarity Less non-polar (moderately hydrophobic) More non-polar (highly hydrophobic)...

by Dr. Yashashwini Reddy | Aug 8, 2025
What Quality Really Means for Pharmaceuticals In the pharmaceutical industry, quality means ensuring that every medicine is safe, effective, and consistent, from raw materials to the final product delivered to patients. It is not just about meeting specifications —...

by Dr. Yashashwini Reddy | Aug 8, 2025
Possible Causes of Out of Specification (OOS) Results OOS results occur when the test results fall outside the predefined acceptance criteria or specification limits. Causes can be grouped into three main categories: 1. Laboratory Errors Instrumental issues:...







