
by Dr. Yashashwini Reddy | Aug 27, 2025
Activated Carbon Filter in Water System 1. Introduction Activated Carbon (AC) filters are widely used in Purified Water (PW) and Water for Injection (WFI) pre-treatment systems.They are installed after multimedia filters and before softeners/RO systems. Main function:...

by Dr. Yashashwini Reddy | Aug 27, 2025
Validation of Clean Room Pass Boxes 1. Introduction A pass box is an enclosed space with doors on both sides, installed between two areas of different cleanliness grades.It allows the transfer of materials with minimum air turbulence and controlled contamination...
by Dr. Yashashwini Reddy | Aug 27, 2025
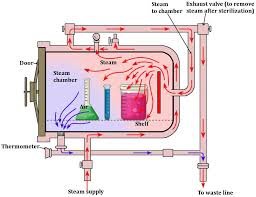
Principle and Working of Autoclave 1. Principle The autoclave works on the principle of moist heat sterilization using saturated steam under pressure. When pressure is applied to water, its boiling point increases. At 121 °C (15 psi / 1.05 kg/cm² above atmospheric...
by Dr. Yashashwini Reddy | Aug 18, 2025
How to Write a Validation Master Plan (VMP) A Validation Master Plan (VMP) is a high-level document that outlines a company’s overall philosophy, intentions, and approach for establishing performance qualification, validation, and compliance of its facilities,...

by Dr. Yashashwini Reddy | Aug 18, 2025
Recovery Factor Determination in Cleaning Validation Definition:Recovery Factor (RF) is the percentage of analyte recovered from a surface during cleaning validation studies. It helps to account for possible losses during swabbing/rinsing and analytical testing,...







