by Dr. Yashashwini Reddy | Sep 6, 2025
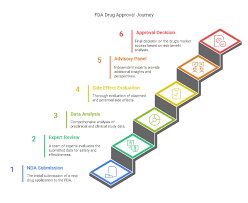
5 Steps of FDA Approvals The U.S. Food and Drug Administration (FDA) follows a structured process to ensure that drugs are safe, effective, and high-quality before they reach patients. 1. Preclinical Testing (Laboratory & Animal Studies) Conducted before human...

by Dr. Yashashwini Reddy | Sep 6, 2025
10 Step Guide to cGMP Certification cGMP (Current Good Manufacturing Practices) certification demonstrates that a company’s facilities, processes, and quality systems comply with international regulatory standards (FDA, EMA, WHO, PIC/S). It assures product quality,...

by Dr. Yashashwini Reddy | Sep 6, 2025
1.0 Purpose To lay down the procedure for the safe, secure, and documented transfer of finished goods from the production/packing area to the bonded store room under controlled conditions to ensure compliance with regulatory and company requirements. 2.0 Scope This...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To establish a uniform procedure for the operation, cleaning, and maintenance of the conveyor belt used in the packing section to ensure smooth functioning and compliance with cGMP requirements. 2.0 Scope This SOP applies to all conveyor belts used in the...

by Dr. Yashashwini Reddy | Sep 2, 2025
How to calculate To calculate the capacity of tablet coating pan we need two factors which are given as below, Brimful volume of pan Tablet bulk density Bring full volume is the volume of the water which can be added in the tablet coating pan up to its brim or upper...







