
by Dr. Yashashwini Reddy | Aug 8, 2025
What Quality Really Means for Pharmaceuticals In the pharmaceutical industry, quality means ensuring that every medicine is safe, effective, and consistent, from raw materials to the final product delivered to patients. It is not just about meeting specifications —...

by Dr. Yashashwini Reddy | Aug 8, 2025
Possible Causes of Out of Specification (OOS) Results OOS results occur when the test results fall outside the predefined acceptance criteria or specification limits. Causes can be grouped into three main categories: 1. Laboratory Errors Instrumental issues:...

by Dr. Yashashwini Reddy | Aug 8, 2025
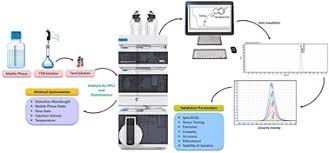
Why Analytical Method Validation is Required? Analytical Method Validation is essential to ensure that a test method is reliable, reproducible, and suitable for its intended purpose.It is required because: Regulatory Compliance Required by ICH Q2(R1), USFDA, EMA, and...

by Dr. Yashashwini Reddy | Aug 8, 2025
User Requirement Specification (URS) of Equipment 1. Definition The URS is a document prepared by the end user that clearly defines the functional, operational, and regulatory requirements an equipment must meet before procurement or qualification.It serves as a...
by Dr. Yashashwini Reddy | Aug 8, 2025
Steps for HPLC Method Development 1. Define the Purpose Identify the analyte(s) to be separated and quantified. Define method requirements (accuracy, precision, sensitivity, run time). 2. Gather Information Chemical structure, polarity, pKa, solubility of the analyte....







