
by Dr. Yashashwini Reddy | Aug 18, 2025
Validation of Compressed Air in Pharmaceuticals Compressed air is widely used in pharmaceutical manufacturing for processes like cleaning, drying, aeration, packaging, and equipment operation. Since compressed air may directly or indirectly come in contact with...

by Dr. Yashashwini Reddy | Aug 18, 2025
📘 Preparation of Master Formula Record (MFR) A Master Formula Record (MFR) is a controlled document that serves as a blueprint for manufacturing a pharmaceutical product. It ensures product quality, consistency, and compliance with regulatory requirements. 🔑 Key Steps...

by Dr. Yashashwini Reddy | Aug 12, 2025
Change Control in Pharmaceuticals Definition:Change control is a formal GMP-compliant system used to manage and document any change in processes, equipment, materials, methods, documents, or facilities to ensure that product quality, safety, and regulatory...
by Dr. Yashashwini Reddy | Aug 9, 2025
Non-conformance in Pharmaceuticals Definition:Non-conformance refers to any deviation from established standards, specifications, regulatory requirements, or approved procedures in the pharmaceutical manufacturing, testing, or distribution process. Examples of...
by Dr. Yashashwini Reddy | Aug 9, 2025
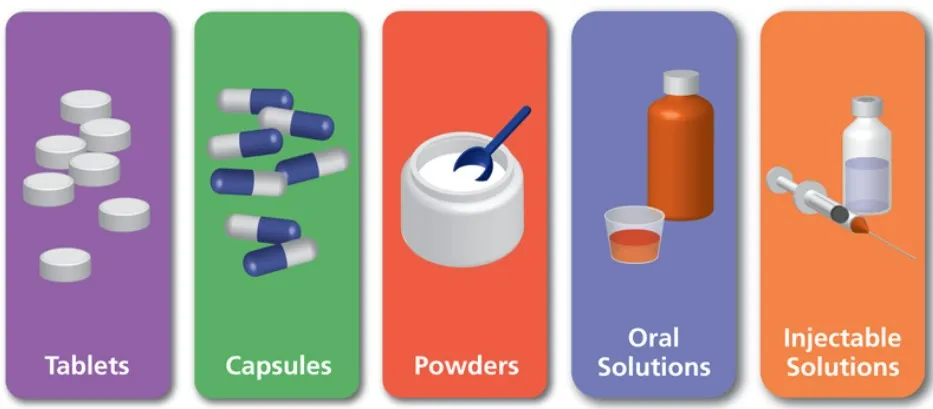
The Shape of Pharmaceutical Dosage Forms Definition:In pharmaceuticals, dosage form shape refers to the physical appearance and geometry of the medicine, which can influence patient compliance, swallowing ease, identification, and manufacturing efficiency. 1. Solid...







