by Dr. Yashashwini Reddy | Sep 23, 2025
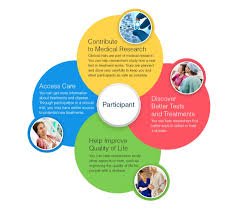
🔹 For Patients Access to New Treatments Early Patients may gain access to investigational drugs, biologics, or devices before they’re widely available. This can be especially important when current treatments are limited or ineffective. Closer Medical Monitoring...
by Dr. Yashashwini Reddy | Sep 20, 2025
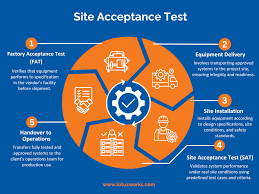
1. Objective To ensure that equipment, system, or instrument is installed correctly at the user site and performs as per the predefined specifications, operational requirements, and approved protocols. 2. Scope Applicable to all new equipment, utilities, systems, and...

by Dr. Yashashwini Reddy | Sep 20, 2025
1. Purpose To establish a documented procedure to verify that the equipment/system has been properly installed according to manufacturer’s specifications, approved drawings, and regulatory requirements. 2. Scope This SOP applies to all new equipment, instruments,...
by Dr. Yashashwini Reddy | Sep 19, 2025
Water for Injection (WFI) in Pharmaceuticals Definition Water for Injection (WFI) is the highest purity water used in the pharmaceutical industry. It meets pharmacopeial standards (USP, EP, IP, JP) and is free from pyrogens, endotoxins, and microbial contamination....
by Dr. Yashashwini Reddy | Sep 18, 2025
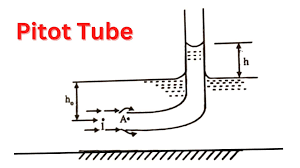
📌 Pitot Tube | Insertion Flow Meter A Pitot tube (also called an Insertion Flow Meter) is a simple, low-cost device used to measure the fluid velocity in pipelines, ducts, or open channels. It works on the principle of differential pressure measurement between...







