
by Dr. Yashashwini Reddy | Oct 9, 2025
In pharmaceuticals, specifications are a set of standards, tests, analytical procedures, and acceptance criteria that define the quality requirements for materials and products. They ensure that every product consistently meets its intended safety, efficacy, and...
by Dr. Yashashwini Reddy | Sep 29, 2025
Advantages of Pharmaceutical Quality Audits Regulatory Compliance – Ensures adherence to GMP, FDA, EMA, WHO, and other regulatory requirements. Risk Identification – Detects potential risks, deviations, and non-compliance before they become critical issues. Continuous...

by Dr. Yashashwini Reddy | Sep 29, 2025
In the pharmaceutical industry, quality audits are critical for ensuring compliance with Good Manufacturing Practices (GMP), regulatory guidelines, and internal quality standards. The main types of audits include: Types of Quality Audits in Pharma Internal Audit...
by Dr. Yashashwini Reddy | Sep 24, 2025
Case Study: Ranbaxy & Glass Particles Recall (2014) Background:In 2014, Ranbaxy Laboratories recalled several batches of injectable products in the United States due to the presence of glass particles in vials. The recall was classified as a Class II recall by the...
by Dr. Yashashwini Reddy | Sep 23, 2025
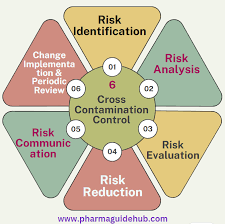
🧪 Case Study: Cross-Contamination in a Multi-Product Pharmaceutical Facility 📌 Background A European pharmaceutical manufacturer operated a multi-product solid oral dosage plant. During a routine EMA inspection, regulatory authorities found traces of a potent API...






