
by Dr. Yashashwini Reddy | Oct 9, 2025
Continuous Manufacturing (CM) is an advanced pharmaceutical production approach where input materials are continuously fed into the system and the processed output (finished product) is continuously removed. Unlike traditional batch manufacturing, which occurs in...
by Dr. Yashashwini Reddy | Aug 10, 2025
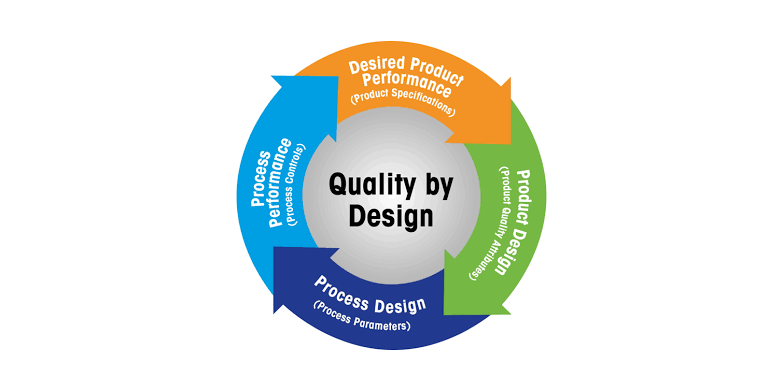
Quality by Design (QbD) in Pharmaceuticals Definition:Quality by Design (QbD) is a systematic, risk-based, proactive approach to pharmaceutical development that emphasizes designing and building quality into the product from the very beginning, rather than relying...

by Dr. Yashashwini Reddy | May 3, 2025
Improving pharmaceutical productivity and product quality is essential to meet the growing global demand for effective and safe medications. This can be achieved through a combination of strategies that address various aspects of pharmaceutical manufacturing, from raw...

by Dr. Yashashwini Reddy | May 1, 2025
Quality by Design (QbD) in Pharmaceuticals: A Detailed Explanation Quality by Design (QbD) is a systematic approach to pharmaceutical development that aims to ensure the quality of a drug product through the design and control of the manufacturing process. This...

by Dr. Yashashwini Reddy | May 1, 2025
Requirements and Implementation of Continuous Training in Pharmaceuticals Continuous training in the pharmaceutical industry is critical for maintaining high standards of quality, ensuring compliance with regulatory guidelines, and keeping employees updated on the...







