by Dr. Yashashwini Reddy | Aug 11, 2025
Calibration of Centrifuge Apparatus in Pharmaceuticals 1. Introduction A centrifuge is used to separate substances of different densities under high-speed rotation. In pharmaceutical laboratories, it plays a critical role in sample preparation, microbial assays,...
by Dr. Yashashwini Reddy | Aug 10, 2025
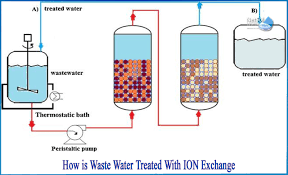
Use of Ion Exchange Resins in Water Purification Systems Ion exchange resins are insoluble, cross-linked polymer beads with functional groups that can exchange specific ions from water, effectively removing unwanted dissolved salts, minerals, or contaminants. They are...
by Dr. Yashashwini Reddy | Aug 10, 2025
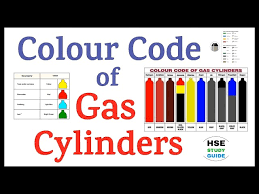
Color Codes for Gas Cylinders in Pharmaceutical Industry In pharmaceuticals, gases are used for instrument calibration, analytical testing, manufacturing processes, and quality control.Each gas cylinder has a standardized color code to quickly identify its contents...
by Dr. Yashashwini Reddy | Aug 10, 2025
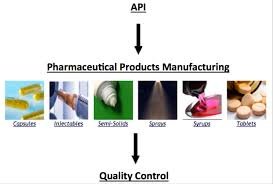
In-process control (IPC) methods are essential in the manufacturing of Active Pharmaceutical Ingredients (APIs) to ensure consistent quality, safety, and compliance with regulatory standards. These methods involve monitoring and adjusting various parameters throughout...
by Dr. Yashashwini Reddy | Aug 9, 2025
Common Causes of Low Quality in Pharmaceuticals Ensuring high-quality pharmaceuticals is crucial to patient safety, regulatory compliance, and brand reputation. Low-quality products can lead to therapeutic failure, adverse reactions, and recalls. The following are...







