
by Dr. Yashashwini Reddy | Aug 28, 2025
1.0 Purpose To define the procedure for the preparation of disinfectant solutions used in pharmaceutical/biotechnology facilities for cleaning and sanitization purposes. 2.0 Scope This SOP applies to all personnel involved in the preparation, dilution, and labeling of...
by Dr. Yashashwini Reddy | Aug 18, 2025
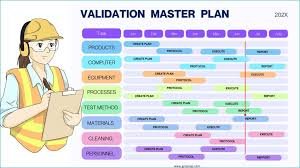
How to Write a Validation Master Plan (VMP) A Validation Master Plan (VMP) is a high-level document that outlines a company’s overall philosophy, intentions, and approach for establishing performance qualification, validation, and compliance of its facilities,...

by Dr. Yashashwini Reddy | Aug 18, 2025
Site Acceptance Test (SAT) The Site Acceptance Test (SAT) is the final phase of equipment/system qualification, conducted at the customer’s site after installation and commissioning. It verifies that the system, machine, or equipment functions correctly in the actual...
by Dr. Yashashwini Reddy | Aug 18, 2025
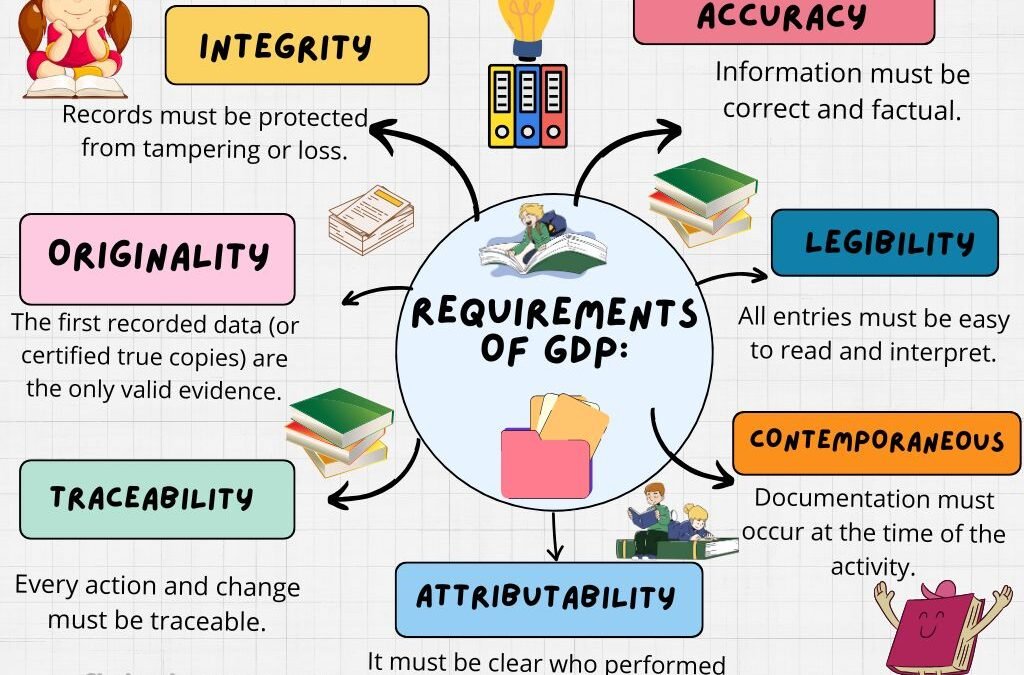
Requirements for Good Documentation Practice (GDP) Legibility All entries should be clear, readable, and permanent (no pencil or erasable ink). Accuracy Data should reflect the actual observation, measurement, or action without manipulation. Contemporaneous Recording...

by Dr. Yashashwini Reddy | Aug 11, 2025
Calibration of Melting Point Apparatus 1. Introduction The melting point of a substance is a critical physical property used in pharmaceuticals to confirm identity, assess purity, and detect impurities. A melting point apparatus must be calibrated to ensure accurate...







