
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Premises and Equipment Use dedicated and clean facilities for production, storage, and filling of gases. Ensure gas cylinders, pipelines, valves, regulators, and manifolds are properly maintained and validated. Prevent cross-contamination between medical and...
by Dr. Yashashwini Reddy | Sep 18, 2025
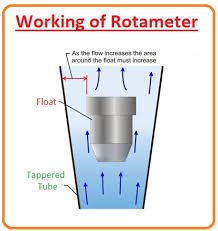
📌 Rotameter A Rotameter is a simple, reliable device used to measure the flow rate of liquids and gases in pharmaceutical, chemical, and other industries. It belongs to the variable area flowmeter family. 🔬 Principle of Rotameter Works on the variable area principle:...

by Dr. Yashashwini Reddy | Sep 11, 2025
📌 Computerized System Validation (CSV) in Pharmaceuticals Definition:Computerized System Validation (CSV) is a documented process of ensuring that computerized systems used in GxP (Good Practice) environments consistently function as intended and comply with...

by Dr. Yashashwini Reddy | Sep 9, 2025
🧫 Typical Microbiology Concerns in an FDA Inspection 1. Environmental Monitoring (EM) Deficiencies Inadequate EM program for cleanrooms and controlled areas. Failure to establish alert/action limits based on historical data. Poor trending and lack of investigation of...
by Dr. Yashashwini Reddy | Sep 9, 2025
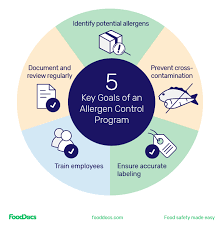
🧾 Allergen Control Plan for Pharmaceuticals 1. Risk Assessment Identify allergenic excipients (e.g., lactose, soy lecithin, egg-derived albumin, peanut oil, gluten, gelatin). Assess risk of cross-contamination during manufacturing, packaging, storage, and cleaning....







