by Dr. Yashashwini Reddy | Sep 15, 2025
GMP Audit Checklist – Storage of Starting Materials 1. Storage Area Design & Conditions Is the storage area clean, well-lit, pest-free, and secure? Are temperature and humidity continuously monitored and recorded? Are conditions maintained as per material storage...

by Dr. Yashashwini Reddy | Sep 15, 2025
Analytical Balances Drift and Its Importance 1. What is Analytical Balance Drift? Analytical balance drift refers to the gradual and unintentional change in the displayed weight reading of a balance over time, even without adding or removing material. This drift can...

by Dr. Yashashwini Reddy | Sep 15, 2025
Major Audit Findings about Equipment and Instruments in Pharmaceuticals Equipment and instruments are critical for ensuring product quality, reliability of test results, and compliance with cGMP. Regulatory inspections (FDA, EMA, MHRA, WHO) often highlight...
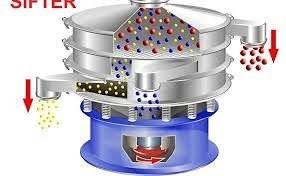
by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To define the procedure for operation, cleaning, and maintenance of the Vibro Sifter used in production to ensure efficient sieving of raw materials and granules as per cGMP requirements. 2.0 Scope This SOP applies to the Vibro Sifter installed in the...
by Dr. Yashashwini Reddy | Sep 1, 2025
⚙️ Equipment Lubricants in Pharmaceuticals In pharmaceutical manufacturing, lubricants are essential for smooth operation of equipment like tablet compression machines, granulators, blenders, filling lines, and packaging machinery. However, because of the high...




