by Dr. Yashashwini Reddy | Sep 9, 2025
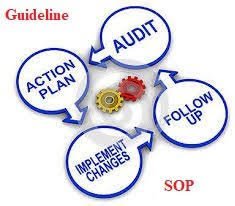
✅ Areas to Inspect During Self-Inspection in Pharmaceuticals Personnel & Training Verify staff qualifications, training records, and adherence to GMP practices. Premises & Facilities Check cleanliness, maintenance, pest control, HVAC systems, and environmental...
by Dr. Yashashwini Reddy | Sep 8, 2025
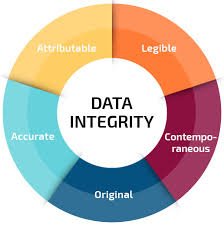
Data Integrity – A Major Problem in Pharmaceuticals Data integrity has become one of the most critical issues in the pharmaceutical industry, directly impacting patient safety, product quality, and regulatory trust. Regulatory agencies like the FDA, EMA, and MHRA...

by Dr. Yashashwini Reddy | Sep 8, 2025
7 Steps for Monitoring Compliance in Pharmaceuticals 1. Establish a Robust Quality Management System (QMS) Build SOPs, policies, and guidelines aligned with FDA, EMA, and ICH standards. Ensure document control and version management. 2. Conduct Regular Internal Audits...

by Dr. Yashashwini Reddy | Sep 8, 2025
Drug recalls can harm patients, damage reputation, and lead to regulatory penalties. To minimize risks, pharmaceutical companies should adopt a proactive quality and compliance strategy. 1. Strong Quality Management System (QMS) Implement robust SOPs across...

by Dr. Yashashwini Reddy | Sep 6, 2025
10 Step Guide to cGMP Certification cGMP (Current Good Manufacturing Practices) certification demonstrates that a company’s facilities, processes, and quality systems comply with international regulatory standards (FDA, EMA, WHO, PIC/S). It assures product quality,...







