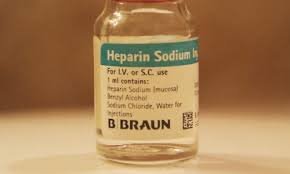
by Dr. Yashashwini Reddy | Sep 24, 2025
📌 Case Study: Heparin Contamination (2008) Background Heparin is a widely used anticoagulant, often derived from porcine intestinal mucosa. In 2007–2008, reports of severe adverse reactions and deaths were linked to contaminated heparin products, especially in the...
by Dr. Yashashwini Reddy | Sep 23, 2025
🧪 Case Study: Cross-Contamination in a Multi-Product Pharmaceutical Facility 📌 Background A European pharmaceutical manufacturer operated a multi-product solid oral dosage plant. During a routine EMA inspection, regulatory authorities found traces of a potent API...

by Dr. Yashashwini Reddy | Sep 23, 2025
📝 Case Study: OOS Investigation – Tablet Dissolution 📍 Background A marketed immediate-release tablet showed dissolution failure during routine quality control testing. Specification: NLT (Not Less Than) 80% drug release in 30 minutes. Observed result: 60–65% release...
by Dr. Yashashwini Reddy | Sep 22, 2025
WHO Guidance on Cross-Contamination Definition:Cross-contamination is the unintended presence of one product (or its residues, microbes, cleaning agents, etc.) in another product. Prevention Measures (as per WHO TRS, GMP guidelines): Dedicated facilities or equipment...
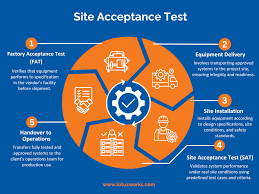
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Objective To ensure that equipment, system, or instrument is installed correctly at the user site and performs as per the predefined specifications, operational requirements, and approved protocols. 2. Scope Applicable to all new equipment, utilities, systems, and...






