by Dr. Yashashwini Reddy | Oct 9, 2025
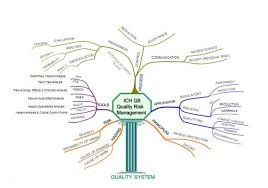
Quality Risk Management (QRM) is a systematic process for the assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. The purpose of QRM is to ensure that product quality, patient safety, and regulatory...
by Dr. Yashashwini Reddy | Oct 8, 2025
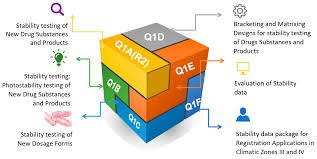
📘 ICH Q1: Stability Testing 🔹 Purpose The ICH Q1 series provides guidance on how to design stability studies to ensure that drug substances and drug products maintain their quality, safety, and efficacy throughout their shelf life under various environmental...

by Dr. Yashashwini Reddy | Oct 8, 2025
🧭 Quality Guidelines – Overview Quality Guidelines are internationally harmonized standards developed mainly by the International Council for Harmonisation (ICH) and other regulatory authorities (like FDA, EMA, WHO, CDSCO).They help ensure that pharmaceutical products...

by Dr. Yashashwini Reddy | Sep 26, 2025
📌 Case Study: Sandoz Canada (2012) – Supply Shortages due to GMP Non-Compliance Background Company: Sandoz Canada, a division of Novartis Year: 2012 Issue: Nationwide shortage of injectable drugs in Canada and USA Cause: Non-compliance with Good Manufacturing...
by Dr. Yashashwini Reddy | Sep 26, 2025
📌 Case Study: Ranbaxy Laboratories – Data Falsification & GMP Violations Background:Ranbaxy Laboratories, once India’s largest generic drug manufacturer, faced one of the biggest regulatory scandals in the pharmaceutical industry. In 2013, the company admitted to...







