
by Dr. Yashashwini Reddy | May 13, 2025
Standard Operating Procedure (SOP) Here is a complete Standard Operating Procedure (SOP) for the Cleaning of Clean Area (Sterile Area) in pharmaceutical manufacturing, ensuring compliance with GMP and sterile environmental control requirements. 1.0...

by Dr. Yashashwini Reddy | May 13, 2025
Standard Operating Procedure (SOP) Here is a complete Standard Operating Procedure (SOP) for the Sanitization of FFS (Form-Fill-Seal) Area, ensuring compliance with GMP standards and maintaining environmental control in sterile and non-sterile...
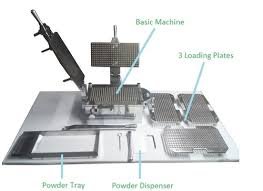
by Dr. Yashashwini Reddy | May 8, 2025
Standard Operating Procedure (SOP) This SOP ensures the cubicle remains clean, compliant with GMP standards, and ready for subsequent production batches. 1. Objective: To lay down the procedure for the proper cleaning of the capsule filling...
by Dr. Yashashwini Reddy | Nov 24, 2024
Understanding Dirty Hold Time in Pharmaceutical Equipment Cleaning Dirty Hold Time (DHT) refers to the duration during which pharmaceutical equipment remains uncleaned after completing a manufacturing process. This period begins when the production ends and ends...
by Dr. Yashashwini Reddy | Oct 21, 2024
Identifying the Worst Case in Cleaning Validation In cleaning validation, determining the worst-case scenario is essential to ensure that the cleaning procedures effectively remove the most challenging residues from manufacturing equipment. This scenario represents...





