by Dr. Yashashwini Reddy | Aug 18, 2025
Revalidation of Pharmaceutical Processes Revalidation in pharmaceuticals refers to the documented evidence that an established process continues to operate in a state of control and consistently produces a product meeting predetermined specifications and quality...
by Dr. Yashashwini Reddy | Aug 18, 2025
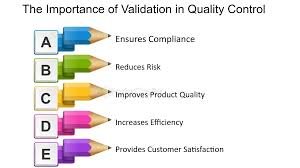
Importance of Validation in Pharmaceuticals Validation in pharmaceuticals is a critical quality assurance tool that ensures products are consistently manufactured to meet predetermined specifications and regulatory requirements. Its importance can be summarized as...
by Dr. Yashashwini Reddy | Aug 12, 2025
Aspect Process Validation Product Validation Definition Documented evidence that a manufacturing process, operated within established parameters, can consistently produce a product meeting its predetermined quality attributes. Documented evidence that a specific...

by Dr. Yashashwini Reddy | Jun 9, 2025
What is a Validation Master Plan (VMP)? A Validation Master Plan is a high-level document that outlines the overall strategy, scope, approach, and responsibilities for validation activities within a pharmaceutical facility. It serves as a roadmap to ensure all...

by Dr. Yashashwini Reddy | Jun 9, 2025
Importance of Validation in Pharmaceuticals Validation in the pharmaceutical industry is a documented process that proves that a system, equipment, method, or process consistently produces a result meeting predetermined quality standards. It is a fundamental part of...




