
by Dr. Yashashwini Reddy | Aug 18, 2025
Cleaning Validation Procedure for Clean-in-Place (CIP) Systems 1. Objective To establish a validated cleaning procedure for Clean-in-Place (CIP) systems ensuring removal of product residues, cleaning agents, and microbial contaminants to acceptable levels. 2. Scope...

by Dr. Yashashwini Reddy | Aug 18, 2025
Sampling in Cleaning Validation in Pharmaceutical Industry Introduction Cleaning validation is a critical part of pharmaceutical manufacturing, ensuring that residues of products, cleaning agents, and microbial contaminants are effectively removed to avoid...
by Dr. Yashashwini Reddy | Aug 18, 2025
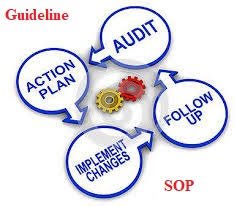
Self-Inspection and Its Implementation in Pharmaceuticals Definition:Self-inspection is a systematic, independent, and documented internal audit conducted by a pharmaceutical company to evaluate compliance with Good Manufacturing Practices (GMP), regulatory...

by Dr. Yashashwini Reddy | Aug 12, 2025
How to Review GMP Documents Like a Pro 1. Understand the Purpose Identify what the document is for (e.g., SOP, batch record, protocol, report). Know the applicable GMP guidelines (US FDA, EMA, WHO, ICH). 2. Check for Compliance Ensure the document aligns with: Current...
by Dr. Yashashwini Reddy | Aug 12, 2025
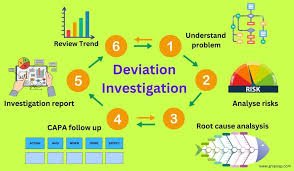
Deviation Control in Pharmaceuticals Definition:A deviation is any departure from approved instructions, standard operating procedures (SOPs), specifications, or established Good Manufacturing Practice (GMP) requirements during manufacturing, testing, packaging,...







