by Dr. Yashashwini Reddy | Aug 27, 2025
🔹 Key Strategies to Strengthen Microbiological Control Raw Material Control Perform supplier qualification and audits. Conduct microbiological testing for bioburden and objectionable organisms. Establish strict acceptance criteria. Water System Management Validate and...

by Dr. Yashashwini Reddy | Aug 18, 2025
Writing Effective SOPs in Pharmaceuticals Standard Operating Procedures (SOPs) are the backbone of pharmaceutical operations, ensuring consistency, compliance, and quality across all processes. An effective SOP must be clear, concise, and compliant with GMP (Good...
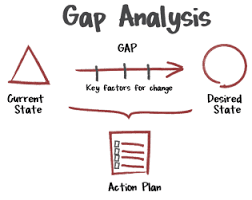
by Dr. Yashashwini Reddy | Aug 18, 2025
Gap Analysis for Regulatory Compliance Gap Analysis is a structured approach used in pharmaceutical and life sciences industries to identify the differences (“gaps”) between current practices and regulatory requirements or industry standards (e.g., FDA, EMA, ICH,...

by Dr. Yashashwini Reddy | Aug 18, 2025
Quality Inspection in Pharmaceuticals Quality inspection in pharmaceuticals is a critical step to ensure that drug products meet regulatory standards, patient safety requirements, and therapeutic efficacy. It involves systematic checks, tests, and verification across...

by Dr. Yashashwini Reddy | Aug 18, 2025
🌟 What Does Quality Really Mean for Pharmaceuticals? In the pharmaceutical industry, quality is far more than just meeting specifications — it is about safeguarding patient health, ensuring regulatory compliance, and maintaining trust. 🔹 Patient Safety First – Every...







