by Dr. Yashashwini Reddy | May 8, 2025
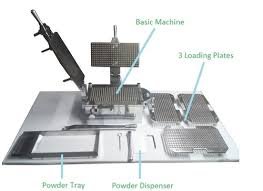
Standard Operating Procedure (SOP) This SOP ensures the cubicle remains clean, compliant with GMP standards, and ready for subsequent production batches. 1. Objective: To lay down the procedure for the proper cleaning of the capsule filling...

by Dr. Yashashwini Reddy | May 3, 2025
Tablet Manufacturing Process: An Overview The tablet manufacturing process is a complex and precise procedure that transforms raw materials into the final pharmaceutical or nutraceutical product. The process involves several key stages, each critical to ensuring the...
by Dr. Yashashwini Reddy | Sep 9, 2024
Wet Granulation Endpoint Determination: Essential Techniques The endpoint of wet granulation is crucial to producing consistent, high-quality granules across batches. Proper control during the addition of the binder solution or solvent ensures granules meet the...
by Dr. Yashashwini Reddy | Aug 26, 2024
“Understanding and Overcoming Picking and Sticking in Tablet Compression” Tablet defects: Sticking and picking are the two most prevalent issues observed during tablet compression, most of you may not differentiate between them due to the similarity, as...





