
by Dr. Yashashwini Reddy | Aug 18, 2025
Cleaning Validation of Pharmaceutical Equipment Cleaning Validation is a documented process that provides a high degree of assurance that equipment used in the manufacturing of pharmaceutical products is consistently cleaned to predetermined and acceptable limits. It...
by Dr. Yashashwini Reddy | Aug 12, 2025
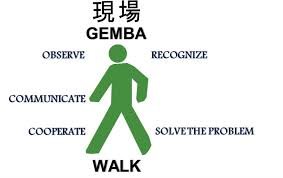
Gemba Walks and Their Implementation in Pharmaceuticals 1. What is a Gemba Walk? Gemba is a Japanese term meaning “the real place”—where work actually happens. In lean manufacturing and quality management, a Gemba Walk is when leaders, managers, or QA personnel go to...
by Dr. Yashashwini Reddy | Aug 12, 2025
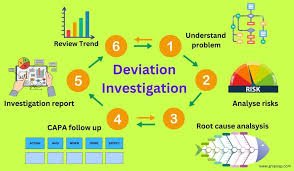
Deviation Control in Pharmaceuticals Definition:A deviation is any departure from approved instructions, standard operating procedures (SOPs), specifications, or established Good Manufacturing Practice (GMP) requirements during manufacturing, testing, packaging,...
by Dr. Yashashwini Reddy | Aug 10, 2025
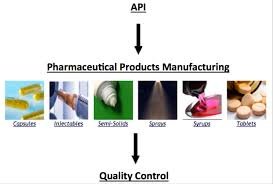
In-process control (IPC) methods are essential in the manufacturing of Active Pharmaceutical Ingredients (APIs) to ensure consistent quality, safety, and compliance with regulatory standards. These methods involve monitoring and adjusting various parameters throughout...
by Dr. Yashashwini Reddy | Aug 9, 2025
Common Causes of Low Quality in Pharmaceuticals Ensuring high-quality pharmaceuticals is crucial to patient safety, regulatory compliance, and brand reputation. Low-quality products can lead to therapeutic failure, adverse reactions, and recalls. The following are...







