
by Dr. Yashashwini Reddy | Sep 15, 2025
Annual Product Quality Review (APQR/APR/PQR) in Quality Improvements 1. What is APQR/APR/PQR? Annual Product Quality Review (APQR), also known as Annual Product Review (APR) or Product Quality Review (PQR), is a regulatory requirement under ICH Q7, EU GMP Chapter 1...
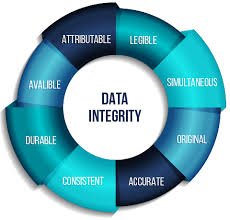
by Dr. Yashashwini Reddy | Sep 15, 2025
Audit Trail Requirements in Pharmaceuticals An audit trail is a secure, computer-generated, time-stamped record that allows for the reconstruction of events related to the creation, modification, or deletion of electronic data. In the pharmaceutical industry, audit...

by Dr. Yashashwini Reddy | Sep 15, 2025
Major Audit Findings about Equipment and Instruments in Pharmaceuticals Equipment and instruments are critical for ensuring product quality, reliability of test results, and compliance with cGMP. Regulatory inspections (FDA, EMA, MHRA, WHO) often highlight...

by Dr. Yashashwini Reddy | Sep 15, 2025
Critical Mistakes During Root Cause Investigation (RCI) Root Cause Investigation (RCI) is an essential step in pharmaceutical quality systems to identify, analyze, and eliminate the underlying causes of deviations, OOS, failures, or non-conformances. However, many...

by Dr. Yashashwini Reddy | Sep 10, 2025
🏭 Guidelines for Preparation of Site Master File (SMF) 📑 1. General Information Company name, address, and contact details. Name of parent company (if applicable). Manufacturing activities performed at the site. Products handled (human, veterinary, APIs, biologicals,...







