
by Dr. Yashashwini Reddy | Aug 10, 2025
Handling of Out of Calibration (OOC) Instruments and Equipment Definition Out of Calibration (OOC) refers to an instrument or equipment that, during calibration verification, fails to meet the specified acceptance criteria or shows results outside its defined...
by Dr. Yashashwini Reddy | Aug 10, 2025
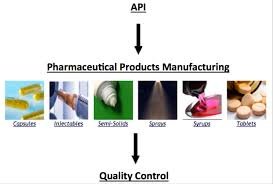
In-process control (IPC) methods are essential in the manufacturing of Active Pharmaceutical Ingredients (APIs) to ensure consistent quality, safety, and compliance with regulatory standards. These methods involve monitoring and adjusting various parameters throughout...

by Dr. Yashashwini Reddy | Aug 9, 2025
Pharmaceutical Compliance and Product Quality 1. Pharmaceutical Compliance Definition:Adherence to all applicable laws, regulations, guidelines, and internal SOPs that govern pharmaceutical manufacturing, testing, storage, and distribution. Key Compliance Areas: GMP...
by Dr. Yashashwini Reddy | Aug 9, 2025
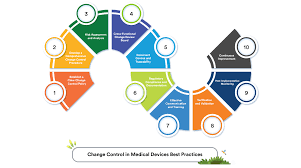
4 Steps to Effective Change Control in Pharmaceuticals 1. Initiation & Documentation Identify the need for change (equipment, process, material, method, etc.). Document the proposed change in a Change Control Form with details like scope, reason, impact area, and...

by Dr. Yashashwini Reddy | Jun 18, 2025
🧠 What Is Pharmacovigilance? Pharmacovigilance (PV) is the science and activities aimed at detecting, assessing, understanding, and preventing adverse effects or any other drug‑related issues once a medicine is in use . The term originates from the Greek pharmakon...







