
by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To lay down the procedure for the operation, cleaning, and maintenance of the paste kettle to ensure uniform binder preparation in compliance with cGMP. 2.0 Scope This SOP applies to the paste kettle used in the production area for binder solution...

by Dr. Yashashwini Reddy | Sep 2, 2025
Generic Drugs Manufacturing: Opportunities and Obstacles Opportunities: Cost-effectiveness: Generic drugs offer patients affordable alternatives to branded medicines, increasing accessibility and market demand. Patent Expiry of Blockbuster Drugs: As patents of many...

by Dr. Yashashwini Reddy | Sep 1, 2025
📘 Preparation of Master Formula Record (MFR) 📌 What is MFR? A Master Formula Record (MFR) is a controlled document that provides the recipe, manufacturing process, equipment, materials, and in-process controls required to produce a pharmaceutical product. It is...
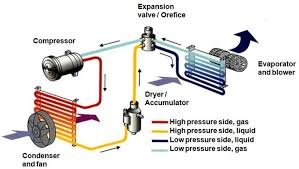
by Dr. Yashashwini Reddy | Sep 1, 2025
🌬️ Basics of HVAC System in Pharmaceuticals 📌 What is HVAC? HVAC = Heating, Ventilation, and Air Conditioning.In pharmaceuticals, HVAC systems are critical to: Maintain controlled environment for manufacturing. Ensure product quality, safety, and regulatory...
by Dr. Yashashwini Reddy | Sep 1, 2025
⚙️ Equipment Lubricants in Pharmaceuticals In pharmaceutical manufacturing, lubricants are essential for smooth operation of equipment like tablet compression machines, granulators, blenders, filling lines, and packaging machinery. However, because of the high...






