by Dr. Yashashwini Reddy | Sep 9, 2025
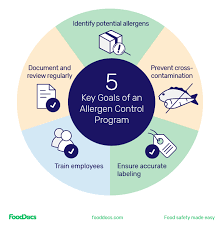
🧾 Allergen Control Plan for Pharmaceuticals 1. Risk Assessment Identify allergenic excipients (e.g., lactose, soy lecithin, egg-derived albumin, peanut oil, gluten, gelatin). Assess risk of cross-contamination during manufacturing, packaging, storage, and cleaning....

by Dr. Yashashwini Reddy | Sep 8, 2025
7 Steps for Monitoring Compliance in Pharmaceuticals 1. Establish a Robust Quality Management System (QMS) Build SOPs, policies, and guidelines aligned with FDA, EMA, and ICH standards. Ensure document control and version management. 2. Conduct Regular Internal Audits...
by Dr. Yashashwini Reddy | Sep 6, 2025
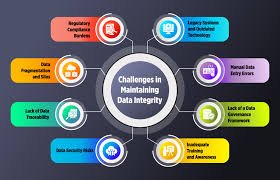
Importance of Data Integrity for Pharmaceutical Regulatory Agencies 1. What is Data Integrity? Data integrity means maintaining the accuracy, completeness, consistency, and reliability of data throughout its lifecycle, ensuring it is attributable, legible,...

by Dr. Yashashwini Reddy | Sep 6, 2025
How to Review GMP Documents Like a Pro 1. Understand the Purpose of the Document Identify the type: SOP, Batch Manufacturing Record (BMR), Protocol, Validation Report, Logbook, etc. Ask: What is this document supposed to control, guide, or prove? 2. Verify Structure...

by Dr. Yashashwini Reddy | Sep 5, 2025
1.0 Purpose To describe the procedure for the operation, cleaning, and maintenance of the Multimill to ensure consistent performance and compliance with cGMP requirements. 2.0 Scope This SOP applies to the Multimill installed in the production area of [Company Name],...







