by Dr. Yashashwini Reddy | Sep 18, 2025
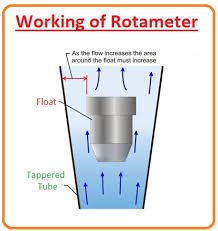
📌 Rotameter A Rotameter is a simple, reliable device used to measure the flow rate of liquids and gases in pharmaceutical, chemical, and other industries. It belongs to the variable area flowmeter family. 🔬 Principle of Rotameter Works on the variable area principle:...

by Dr. Yashashwini Reddy | Sep 18, 2025
1. Objective Demonstrate that the proposed purified water generation and distribution system is designed, specified and documented such that, when built and operated as designed, it will consistently produce Purified Water that meets USP requirements and is suitable...

by Dr. Yashashwini Reddy | Sep 16, 2025
1.0 Purpose To describe the procedure for proper cleaning, maintenance, and storage of sampling equipment used for raw materials, intermediates, and finished products to avoid contamination, cross-contamination, and ensure equipment integrity. 2.0 Scope This SOP...

by Dr. Yashashwini Reddy | Sep 15, 2025
Internal Audit / Self-Inspection Checklist (Defects & Regulatory Compliance Focus) 1. Documentation & Records Are SOPs, policies, and work instructions up-to-date, approved, and controlled? Is there evidence of uncontrolled or obsolete documents in use? Are...
by Dr. Yashashwini Reddy | Sep 13, 2025
HR Audit Checklist 1. Recruitment & Selection Existence of recruitment policies and SOPs. Proper manpower requisition approvals before hiring. Background verification and reference checks of employees. Job descriptions and competency requirements documented....







