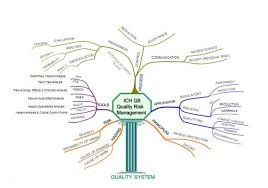
by Dr. Yashashwini Reddy | Oct 9, 2025
Quality Risk Management (QRM) is a systematic process for the assessment, control, communication, and review of risks to the quality of a pharmaceutical product across its lifecycle. The purpose of QRM is to ensure that product quality, patient safety, and regulatory...

by Dr. Yashashwini Reddy | Oct 9, 2025
Good Manufacturing Practices (GMP) for APIs are essential to ensure that the Active Pharmaceutical Ingredients produced are of consistent quality and meet their intended use requirements. These guidelines are mainly described in ICH Q7 – Good Manufacturing Practice...

by Dr. Yashashwini Reddy | Sep 29, 2025
Formats of Pharmaceutical Audits On-Site Audit (Physical Audit) Conducted at the manufacturing site, laboratory, warehouse, or supplier facility. Provides direct observation of processes, equipment, and personnel. Most common format for GMP compliance checks. Remote...
by Dr. Yashashwini Reddy | Sep 29, 2025
Advantages of Pharmaceutical Quality Audits Regulatory Compliance – Ensures adherence to GMP, FDA, EMA, WHO, and other regulatory requirements. Risk Identification – Detects potential risks, deviations, and non-compliance before they become critical issues. Continuous...

by Dr. Yashashwini Reddy | Sep 29, 2025
📋 Types of Audits in the Pharmaceutical Industry 1. Internal Audit (Self-Inspection) Purpose: To assess compliance with internal SOPs and GMP requirements. Conducted By: Company’s own QA or compliance team. Frequency: Regularly scheduled (e.g., annually or quarterly)....






