
by Dr. Yashashwini Reddy | Oct 9, 2025
Elaboration:The development and manufacture of drug substances (Active Pharmaceutical Ingredients – API) encompass all stages from the initial synthesis or isolation of the active compound to its large-scale commercial production under Good Manufacturing Practice...
by Dr. Yashashwini Reddy | Oct 9, 2025
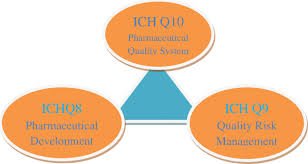
Pharmaceutical development, as described in ICH Q8 (R2), is the process of designing a quality product and its manufacturing process to consistently deliver the intended performance. The goal is to understand how formulation and process variables influence product...
by Dr. Yashashwini Reddy | Aug 10, 2025
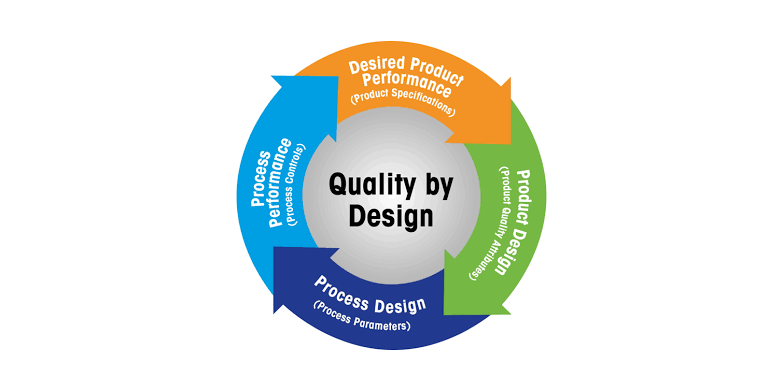
Quality by Design (QbD) in Pharmaceuticals Definition:Quality by Design (QbD) is a systematic, risk-based, proactive approach to pharmaceutical development that emphasizes designing and building quality into the product from the very beginning, rather than relying...

by Dr. Yashashwini Reddy | May 3, 2025
Granule size plays a crucial role in the formulation and performance of tablet-based pharmaceutical products. It significantly affects both the stability of the tablet during its shelf life and the bioavailability of the drug when taken by the patient....

by Dr. Yashashwini Reddy | May 1, 2025
Optimizing the Calibration Process for Dissolution Testing Equipment Dissolution testing is a crucial process in pharmaceutical and biopharmaceutical industries to ensure the release of active ingredients from solid dosage forms (e.g., tablets, capsules) into the...







