by Dr. Yashashwini Reddy | Oct 9, 2025
Analytical procedure development is the process of designing and optimizing methods to accurately and precisely measure the active pharmaceutical ingredient (API), excipients, impurities, and degradation products in a drug substance or drug product. The goal is to...
by Dr. Yashashwini Reddy | Aug 8, 2025
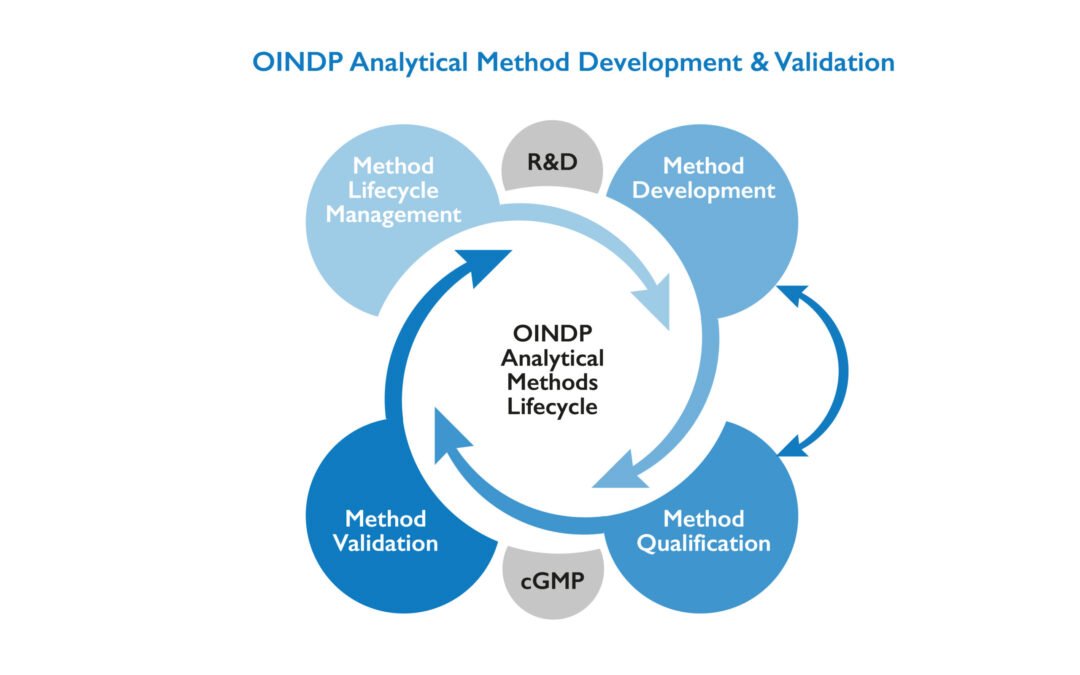
Here’s a clear breakdown of the Steps for Analytical Method Development in pharmaceuticals, following regulatory expectations like ICH Q2(R2): Steps for Analytical Method Development Define the Objective (Method Purpose) Understand what needs to be measured (API,...

by Dr. Yashashwini Reddy | Apr 27, 2025
Standard Operating Procedure (SOP) 1. Purpose This SOP describes the selection criteria and step-by-step procedure for choosing appropriate samples for dissolution profile studies to ensure accurate, reproducible, and scientifically justified...





