by Dr. Yashashwini Reddy | Aug 12, 2025
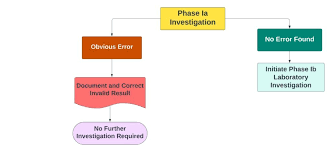
Investigation of OOS Results in Analytical Testing 1. Definition An Out of Specification (OOS) result occurs when an analytical test result for a product, raw material, or intermediate falls outside the approved acceptance criteria or specifications set by regulatory...

by Dr. Yashashwini Reddy | Aug 9, 2025
Out of Specification (OOS) Investigation in Pharmaceuticals Definition OOS results are test results that fall outside the pre-established acceptance criteria defined in specifications, procedures, or regulatory filings.They can occur in raw materials, in-process...

by Dr. Yashashwini Reddy | Aug 8, 2025
Possible Causes of Out of Specification (OOS) Results OOS results occur when the test results fall outside the predefined acceptance criteria or specification limits. Causes can be grouped into three main categories: 1. Laboratory Errors Instrumental issues:...

by Dr. Yashashwini Reddy | Aug 8, 2025
Investigation of OOS Results in Analytical Testing 1. What is an OOS Result? An Out-of-Specification (OOS) result is any analytical test result that falls outside the established acceptance criteria/specifications outlined in an approved method, regulatory filing, or...

by Dr. Yashashwini Reddy | May 19, 2025
A Systematic Investigation of Out-of-Specification (OOS) Results in Analytical Testing is a structured approach to identify, evaluate, and resolve instances where test results fall outside predefined specifications. This process is vital in pharmaceutical quality...







