
by Dr. Yashashwini Reddy | May 18, 2024
Environmental monitoring in a pharmaceutical manufacturing facility 1.0 Purpose This procedure defines the requirements for conducting viable and non-viable environmental monitoring in classified areas of the pharmaceutical manufacturing facility. Environmental...

by Dr. Yashashwini Reddy | May 18, 2024
Cleaning of Glassware for Microbiological Testing 1.0 Objective To establish a comprehensive procedure providing guidelines for effective cleaning and preparation of various types of glassware used for microbiological testing and analysis in the laboratory. 2.0 Scope...
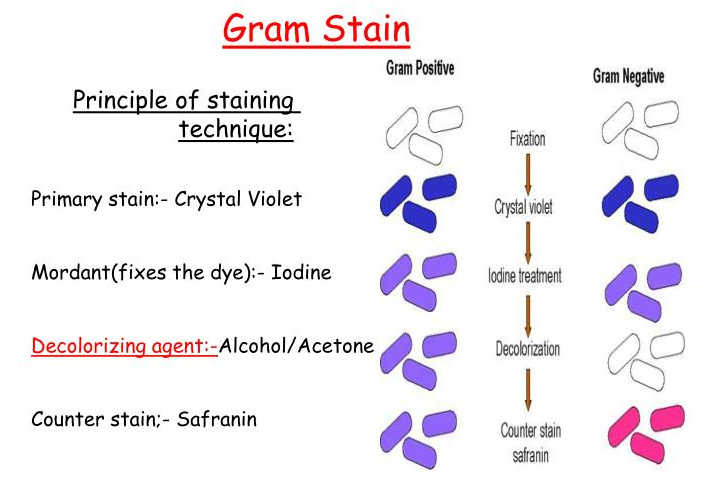
by Dr. Yashashwini Reddy | May 16, 2024
Purpose This SOP describes the procedure for performing the Gram staining technique, which is used to differentiate and identify gram-positive and gram-negative bacteria based on their cell wall characteristics. Scope This procedure applies to all microbiological...
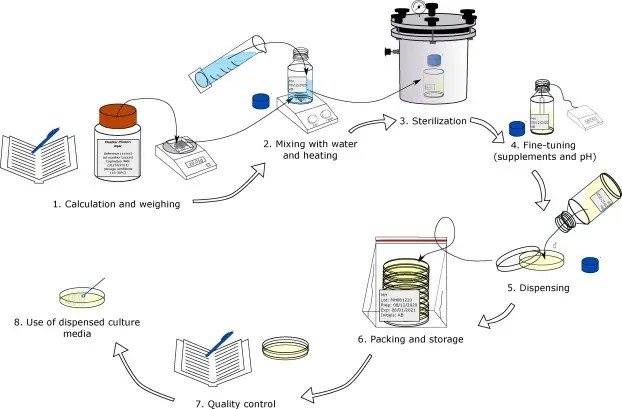
by Dr. Yashashwini Reddy | May 16, 2024
Title: SOP for Preparation, Sterilization, Storage, and Growth Promotion Testing of Solid and Liquid Media 1.0 Objective To provide a standardized method for preparation, sterilization, storage, and growth promotion testing of microbiological media used for microbial...






