
by Dr. Yashashwini Reddy | Oct 8, 2025
🧪 ICH Q2: Analytical Validation 🔹 Full Title: ICH Q2(R2) – Validation of Analytical Procedures 🔹 Objective: To provide guidance on the validation of analytical methods used to test drug substances and products — ensuring that the methods are accurate, reliable, and...
by Dr. Yashashwini Reddy | Aug 9, 2025
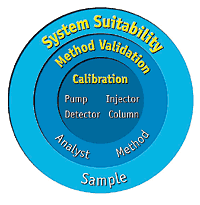
System Suitability in HPLC Analysis What is System Suitability? System Suitability Testing (SST) is a set of analytical checks performed before and during analysis to ensure that the HPLC system and method are capable of producing accurate, precise, and reproducible...

by Dr. Yashashwini Reddy | Aug 8, 2025
Why Analytical Method Validation is Required? Analytical Method Validation is essential to ensure that a test method is reliable, reproducible, and suitable for its intended purpose.It is required because: Regulatory Compliance Required by ICH Q2(R1), USFDA, EMA, and...
by Dr. Yashashwini Reddy | Aug 8, 2025
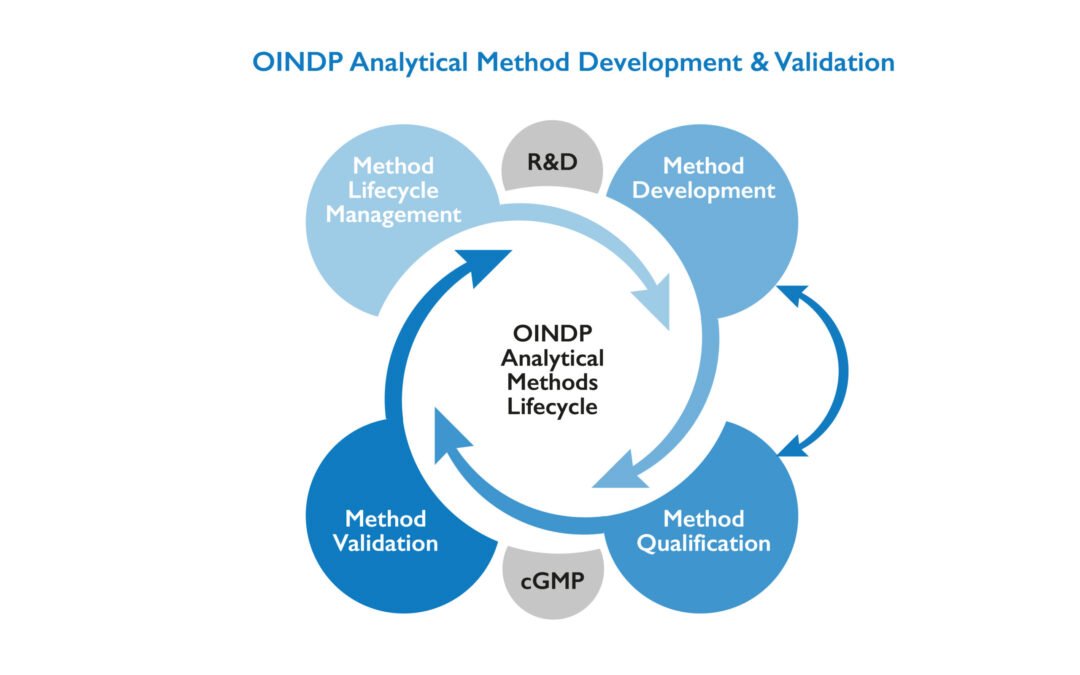
Here’s a clear breakdown of the Steps for Analytical Method Development in pharmaceuticals, following regulatory expectations like ICH Q2(R2): Steps for Analytical Method Development Define the Objective (Method Purpose) Understand what needs to be measured (API,...







