by Dr. Yashashwini Reddy | Oct 9, 2025
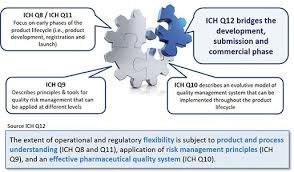
Lifecycle Management (LCM) in the pharmaceutical industry refers to the systematic approach of managing a product from its development phase through commercialization, post-approval changes, and discontinuation. It ensures continuous product quality, compliance, and...
by Dr. Yashashwini Reddy | Jun 3, 2024
Life Cycle Management of Investigational New Drug Application: Life cycle management of IND applications involves activities that ensure compliance with the regulatory requirements and the safe & smooth running of clinical trials. The important things to consider...



