
by Dr. Yashashwini Reddy | Sep 29, 2025
📋 Types of Audits in the Pharmaceutical Industry 1. Internal Audit (Self-Inspection) Purpose: To assess compliance with internal SOPs and GMP requirements. Conducted By: Company’s own QA or compliance team. Frequency: Regularly scheduled (e.g., annually or quarterly)....
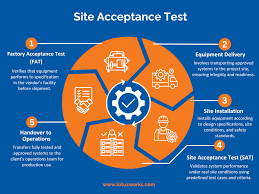
by Dr. Yashashwini Reddy | Sep 20, 2025
1. Objective To ensure that equipment, system, or instrument is installed correctly at the user site and performs as per the predefined specifications, operational requirements, and approved protocols. 2. Scope Applicable to all new equipment, utilities, systems, and...
by Dr. Yashashwini Reddy | Sep 15, 2025
GMP Audit Checklist – Storage of Starting Materials 1. Storage Area Design & Conditions Is the storage area clean, well-lit, pest-free, and secure? Are temperature and humidity continuously monitored and recorded? Are conditions maintained as per material storage...

by Dr. Yashashwini Reddy | Aug 11, 2025
Calibration of Digital Polarimeter in Pharmaceuticals 1. Introduction A digital polarimeter is used to measure the optical rotation of optically active substances, which is essential in pharmaceutical quality control for raw materials (e.g., sugars, amino acids,...

by Dr. Yashashwini Reddy | Aug 11, 2025
Calibration of FTIR Spectrophotometer 1. Introduction The Fourier Transform Infrared (FTIR) spectrophotometer is used to identify and characterize materials by measuring their infrared absorption spectra. Calibration ensures the instrument provides accurate wavelength...






