by Dr. Yashashwini Reddy | Oct 8, 2025
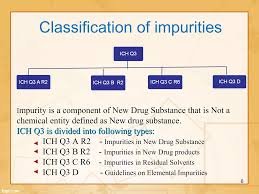
Elaboration:Impurities are unwanted chemicals that remain with the active pharmaceutical ingredient (API) or develop during formulation and storage. According to ICH Q3A (for new drug substances) and ICH Q3B (for new drug products), impurities must be identified,...

by Dr. Yashashwini Reddy | Oct 8, 2025
🧭 Quality Guidelines – Overview Quality Guidelines are internationally harmonized standards developed mainly by the International Council for Harmonisation (ICH) and other regulatory authorities (like FDA, EMA, WHO, CDSCO).They help ensure that pharmaceutical products...
by Dr. Yashashwini Reddy | Aug 8, 2025
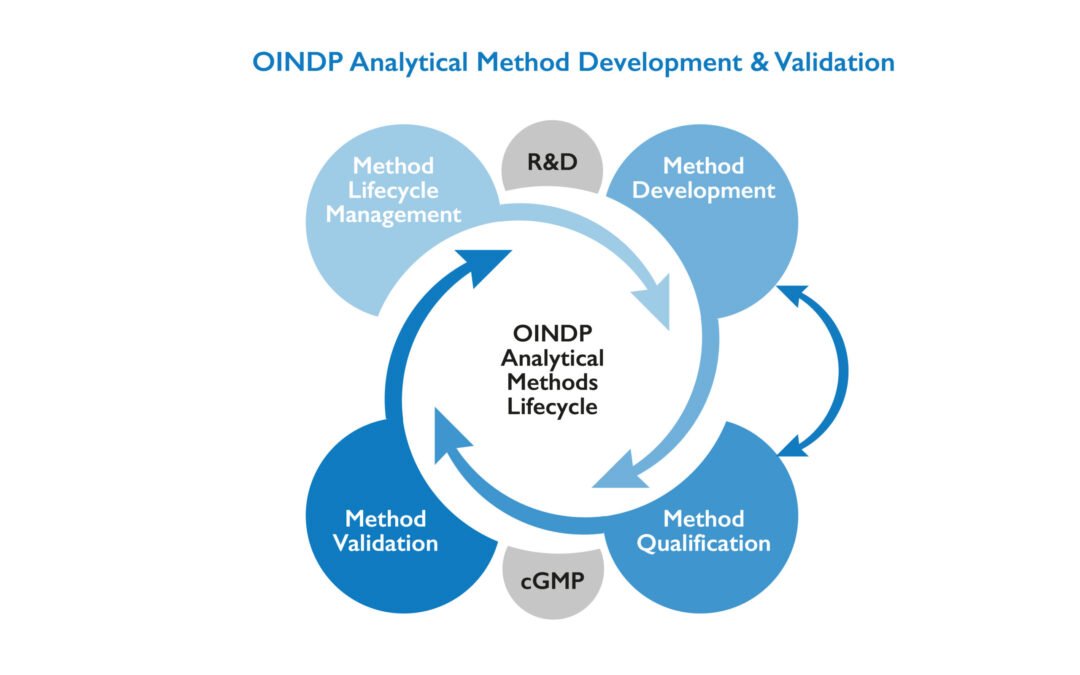
Here’s a clear breakdown of the Steps for Analytical Method Development in pharmaceuticals, following regulatory expectations like ICH Q2(R2): Steps for Analytical Method Development Define the Objective (Method Purpose) Understand what needs to be measured (API,...

by Dr. Yashashwini Reddy | Jun 26, 2025
✅ Pharmacovigilance Audits and Inspections 🔍 1. Pharmacovigilance Audits 📘 Definition: A systematic, independent, and documented process to evaluate the pharmacovigilance (PV) system, its quality system, and compliance with regulatory requirements. 📌...

by Dr. Yashashwini Reddy | Jun 26, 2025
Here is a comprehensive list and explanation of ICH Guidelines related to Pharmacovigilance, which aim to harmonize safety monitoring practices across regulatory regions: ✅ Core ICH Guidelines for Pharmacovigilance Code Title Focus Area E2A Clinical Safety Data...







